The Best Air Purifying Indoor Plants — That Are Also…
Learn which plants won’t irritate your eczema — and can also help purify the air in your home.

Published On: Apr 29, 2022
Last Updated On: Sep 28, 2022
The Oxford Dictionary defines the term “atopic” as “a form of allergy in which a hypersensitivity reaction may occur in a part of the body not in contact with the allergen.” These reactions on the skin cause dermatitis, while reactions in the airway can cause asthma or rhinitis (sometimes called hay fever). After allergen exposure at a site like the skin, some of these immune reactions can be local, such as allergic (contact) dermatitis, but others can happen in the lungs in the case of asthma, gut in the case of food allergies and nose in the case of allergic rhinitis.
Population, statistical and mechanistic studies have repeatedly shown that atopic dermatitis (AD), asthma and allergic rhinitis often co-occur in the same individual.1 One in three children with AD will additionally develop asthma or allergic rhinitis. The risk of developing asthma increases with AD severity as more than 50% of children with severe AD also develop asthma.1 AD patients also have a high incidence of accompanying food allergies. The earlier in life AD begins and the more severe it is, the higher the association with food allergies.2 In a large study using a database of 244,776 AD and matched non-AD patients, the prevalence and incidence of other Type 2 inflammatory diseases (including asthma and rhinitis) more than doubled among children aged 0-2 years with AD compared to those without.3 A recent study of 212 AD patients aged 12-76 indicated that 69% also had rhinitis, 33% also had allergic conjunctivitis (eye-related allergies) and 29% also had asthma.4 The connectivity of AD, food allergies, asthma and rhinitis is often referred to as the atopic march.
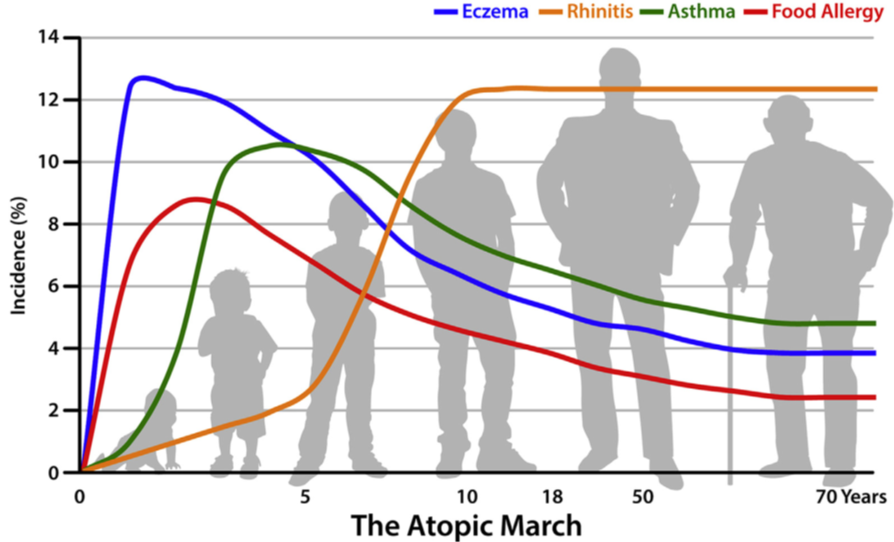
Considerable research has been done to investigate all forms of atopic diseases and how they may be linked together. One concept of how these diseases are interconnected is that disruption of the skin barrier in AD leads to allergen sensitization in the skin, which can trigger inflammation at other epithelial (skin-like) body surfaces including the gastrointestinal tract (food allergy), upper respiratory tract (allergic rhinitis) and lower respiratory tract (asthma).5 It has been long thought that AD is the first of these related diseases to arise followed by a “march” toward the other diseases. Indeed, studies have shown that AD typically has the earliest onset, followed by food allergies, both of which come before the airway diseases (asthma and rhinitis) and all four of which often arise before a person is 5 years-old (Figure 1).6 However, two long-term studies of over 207 and 408 years have led researchers to begin to focus less on the order of disease progression (the march), which might not be as clear-cut as originally thought, and more on the associations between these diseases and genetic and environmental factors that may contribute to the relationships between them.5
Dr. Candrice Heath, of Temple University, said, “We are taught very early in our medical training that AD, allergic rhinitis and asthma are part of the atopic triad. Explaining to parents that their child with AD may go on to develop asthma or allergic rhinitis is commonplace. However, recent research suggests that a patient’s demographics and genetics may be associated with a specific trajectory of the atopic march from AD to other conditions. To help us better understand exactly how these diseases are linked, it is important that patients share information about their experiences with AD and the atopic march with clinicians and researchers. Our current research conclusions may focus only on associations, but we hope that in the future we may be able to make specific predictions about what to expect in one’s personal journey with AD.”
Research continues to examine what causes atopic reactions that originate in the skin and also occur at multiple body sites. Dr. Lawrence Eichenfield, at the University of California San Diego, said, “So much has happened in the field of AD in the past 10 years. We now have better studies that have looked at the course of AD in terms of onset in childhood or beyond the early childhood years, persistence and spontaneous resolution, all of which have given us new information into the development of allergies. Milder AD patients have around a 15% rate of having a true food allergy (an allergic clinical reaction consistently on ingestion of a food), while those with more severe AD have a 40% chance of having at least one food allergy. Allergic rhinitis occurs in about a third of individuals with AD within the first few years of life and asthma is very common in children with AD, developing in later childhood. What is not yet known is if earlier aggressive therapy, either topically or systemically, will change the development of these conditions.” New insights into some of the mechanisms are beginning to emerge, including how skin barrier defects, genetics and environmental factors trigger full-body immune responses.
In AD the skin barrier is disrupted and skin exposure to allergens can induce the skin’s immune response. Mechanistic links between AD, allergen exposure and asthma involve a molecule called thymic stromal lymphopoietin (TSLP) that is secreted by keratinocytes, the main cells that make up the skin structure. TSLP secretion by keratinocytes after allergen exposure has been shown to sensitize mice9-11 and humans12 to asthma. TSLP inhibitors are under development as a therapy for asthma12 and TSLP is among the targets of biologic-based therapies also being studied for AD.13 Links between AD and food allergy have been studied in mice, showing that application of egg or peanut allergens onto skin with a barrier defect increased the presence of allergy-related immune signals like the antibody IgE and TSLP.14 In humans, increased loss of water from the skin (trans-epidermal water loss) at two days of life was predictive of food allergy development at the age of 2 years-old.14 Current research supports a significant link between the skin and its protective barrier and the development of immune reactions that can promote body-wide allergic responses leading to other atopic diseases.
The most widely studied genetic mutation in AD is filaggrin, which is also associated with increased risk for asthma with or without association with AD.15Filaggrin mutations are also associated with increased risk of food allergy.15 A recent study found 227 different types of filaggrin variants or mutations associated with asthma, atopic dermatitis, rhinitis and other atopic diseases.16
A few studies have also examined common genetic alterations that can be found in AD, asthma and allergic airway disease using a genome-wide approach, and a few genetic factors were found to be in common between all these atopic diseases.15 The contribution of personal (race, ethnicity) and genetic factors influencing associations between atopic march diseases has also been investigated. A recent study used computer programming to analyze 15 years of medical records (2001-2016) from a cohort of 158,510 children who are part of the Children’s Hospital of Philadelphia network, combined with a large genetic study called a Genome Wide Association Study (GWAS).17 The study sought to identify associations between atopic diseases and other demographic information like race or ethnicity from the medical records and subsequently try to determine genetic components influencing these associations.17
Self-identified Black race associated with progression from AD to asthma. Asian or Pacific Islander race associated with progression from AD to allergy (IgE)-mediated food allergy. White race associated with progression from AD to rhinitis. The study also pinpointed specific genetic differences between individuals of African and European ancestry that correlated with the type of allergic disease an individual may go on to exhibit.17 Another analysis of 8014 Black (52%) and white (37.7%) children with AD found that children with the longest duration of AD had greater levels of other allergic diseases, but that there was not one progressive pathway from one disease to another.18 Together, these results suggest that there is not one overall atopic march, but potentially many depending on individual identities.
Research like this evaluates an individual’s characteristics in order to better understand what a patient’s disease course might look like and guide treatment decisions. However, Dr. Heath cautioned, “I believe that racial self-identification and genetic mutations do not tell the entire story of the atopic march. Health inequities, environmental factors and living conditions impact all AD patients and likely the atopic march. A multidisciplinary approach to looking at this big picture is required.”
Since inflammation is the body’s defense against infection or injury, it is tightly regulated and there are different types of immune reactions involving different cells and different signals between cells (called cytokines or interleukins). Type 1 immunity protects against microbes inside cells, Type 2 immunity protects against parasites and Type 3 immunity protects against bacteria and fungi outside of cells.19 However, all three of these beneficial immune reaction types can also cause diseases if they inappropriately respond to the wrong things and become dysregulated. For example, Types 1 and 3 immunity can underlie diseases such as rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease, among others. Cells involved in Type 2 immunity produce interleukins (IL-4, IL-5, IL-9 and IL-13) and an antibody called IgE, all of which are involved in allergic reactions such as mucus secretion, airway reduction, itchiness and redness of the skin and eyes and other symptoms. All the interrelated atopic march reactions and diseases are part of the Type 2 immune response.19
Further, the types of immune reactions can differ between children and adults with a more complex range of cells and cytokine signals involved in childhood compared to adulthood AD.6 While AD symptoms may disappear after childhood, an adult patient who had AD may still be at risk for the other atopic conditions to develop throughout life. New treatments under development for atopic diseases target various components of Type 2 inflammation including specific biologics like dupilumab which is approved for AD, asthma and chronic rhinosinusitis (targets IL-4)20, tralokinumab (targets IL-13), oral and topical JAK inhibitors and other biologics under development to target various components of the Type 2 immunity pathway (other interleukins and IgE).21-23
An individual’s environment from birth onward can also be associated with whether they are at higher risk to develop allergic diseases. The method of birth (vaginal vs. Cesarean section) is the first factor that influences the establishment of the entire system of bacteria and other organisms that live on our skin, in our airways and in our gut (called the microbiome). The microbiome and the immune system develop together in infants, so the microbiome impacts a child’s immune system for life. Diet and early exposure to medicines such as antibiotics can also influence the microbiome and formation of immune reactions.24 A recent study of medical records of 158,510 births found associations between vaginal delivery, exclusive breastmilk feeding and reduced cumulative allergic burden. Antibiotic and antacid exposure associated with increased cumulative allergic burden during childhood.24 The delicate balance of the microbiome is important in development of food allergies with a direct correlation between Staphylococcus aureus colonization and sensitization to eggs or peanuts, regardless of AD severity.25 Early and frequent exposure to environmental allergens (dust, pet dander) can also result in rhinitis and asthma.26
AD, food allergies, asthma and rhinitis all typically arise early in life, before 5 years-old.6 However, associations between AD and these other atopic comorbid diseases can remain into adulthood.27 Additionally, while incidence of AD, asthma and food allergy symptoms decrease to below 5% of the adult population, the types of intersecting comorbidities expand to include eosinophilic chronic rhinosinusitis (Figure 1).6
Figure 1: A graphical representation of timing and overlap of the various related immune-mediated allergic diseases eczema (AD) in blue, food allergy in red, asthma in green and rhinitis in orange. This shows the generalized time of onset and duration of each disease.6
Much work is being done to understand whether early intervention to prevent AD could lead to reduced incidence of the other atopic march diseases. Researchers are focusing on strategies to facilitate barrier repair, early proactive treatment for AD and reduction in environmental food allergen exposure in the prevention of food sensitization and allergy.2 AD patients are often advised to avoid irritants including soaps and detergents and to use moisturizers to reduce trans-epidermal water loss and improve the skin barrier as an important line of defense against allergen exposure.28 Several emerging therapeutics for AD may work to prevent development of the other atopic diseases, or treat multiple atopic conditions simultaneously.19
Dr. Jiade (Jeff) Yu, of Massachusetts General Hospital said, “AD is much more than skin deep. While AD tends to decrease as kids get older, a subset of children maintains moderate to severe eczema into adulthood. Research should be focused on preventing AD in the first place, and also prevention of the atopic march.I hope that if we know exactly how these co-conditions start, we can prevent or halt progression of the atopic march.”
Dr. Eichenfeld and colleagues published 20 years ago28 that “treatment of AD requires a comprehensive approach that includes evaluation of potential triggers and education of the patient and family regarding proper avoidance measures. Hydration of the skin and maintenance of an intact skin barrier remain integral to proper management. Studies aimed at defining optimal combination therapy and early intervention might change the treatment paradigm for AD.” When asked how he feels these truths have changed over the years he responded, “I think the statement still holds true. While we’ve markedly improved our armamentarium for treatment of inflammatory disease, we’re just beginning longer-term assessment of how early intervention may impact the disease over time.”
However, not every person that will develop atopic disease seeks medical treatment for AD. In cases of mild AD, the childhood disease may resolve but may later manifest as food allergies, asthma, or rhinitis. Further, AD may never develop – practicing allergists know the considerable variability in the number and sequence of allergic conditions that individuals develop even though the basis for these allergic march pathways, or trajectories, is not yet well understood.29 Patients need holistic approaches to look at the big picture in order to be properly treated no matter whether their initial medical visit is with a dermatologist, pulmonologist, or allergist. New guidelines from the American Academy of Dermatology (AAD) are also helping to bring together information on all the co-morbidities (associated diseases) of AD to be sure that physicians are aware of them and use this information to inform dialogue with patients and care decision-making.5
Additionally, multidisciplinary approaches and centers are being initiated worldwide to bring together experts to help patients navigate the range of atopic co-occurring conditions in one place.20 Dr. Eichenfeld said, “Our experience with multidisciplinary care of patients with AD at Rady Children’s Hospital has been quite rewarding and insightful. Allergy, dermatology, clinical pharmacy and research associates see our patients, and we’ve seen that more comprehensive and holistic management improves the lives of affected individuals and families. We have had experiences with patients who are prescribed a medication for their AD but wind up with tremendous secondary benefits to their asthma. The most exciting research questions relate to whether early intervention can modulate the development and/or course of allergic comorbidities. As our systemic medicines improve, we are looking forward to assessing the impact of treatment on the immunologic status of the individuals, as well as the clinical course.”
Take home points:
References:
1. Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin North Am. 2010;30(3):269-280.
2. Tsakok T, Marrs T, Mohsin M, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. 2016;137(4):1071-1078.
3. Paller AS, Mina-Osorio P, Vekeman F, et al. Prevalence of type 2 inflammatory diseases in pediatric patients with atopic dermatitis: Real-world evidence. J Am Acad Dermatol. 2022;86(4):758-765.
4. Sanclemente G, Hernandez N, Chaparro D, Tamayo L, Lopez A, Colombian Atopic Dermatitis Research G. Epidemiologic features and burden of atopic dermatitis in adolescent and adult patients: A cross-sectional multicenter study. World Allergy Organ J. 2021;14(12):100611.
5. Davis DMR, Drucker AM, Alikhan A, et al. AAD Guidelines: awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol. 2022.
6. Davidson WF, Leung DYM, Beck LA, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: Mechanisms and interventions”. J Allergy Clin Immunol. 2019;143(3):894-913.
7. Gough H, Grabenhenrich L, Reich A, et al. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol. 2015;26(5):431-437.
8. Martin PE, Matheson MC, Gurrin L, et al. Childhood eczema and rhinitis predict atopic but not nonatopic adult asthma: a prospective cohort study over 4 decades. J Allergy Clin Immunol. 2011;127(6):1473-1479 e1471.
9. Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7(5):e1000067.
10. Han H, Xu W, Headley MB, et al. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012;5(3):342-351.
11. Leyva-Castillo JM, Hener P, Jiang H, Li M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2013;133(1):154-163.
12. Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777-792.
13. Pescitelli L, Rosi E, Ricceri F, Pimpinelli N, Prignano F. Novel Therapeutic Approaches and Targets for the Treatment of Atopic Dermatitis. Curr Pharm Biotechnol. 2021;22(1):73-84.
14. Tham EH, Leung DY. Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March. Allergy Asthma Immunol Res. 2019;11(1):4-15.
15. Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. 2017;278(1):116-130.
16. Salama RH, Rasheed Z, Ahmed AA, et al. Missense, silent, non-sense and frame-shift mutations in exon 3 of the filaggrin gene in patients with bronchial asthma, atopic dermatitis, allergic rhinitis and mixed atopy. Nucleosides Nucleotides Nucleic Acids. 2021;40(3):357-367.
17. Gabryszewski SJ, Chang X, Dudley JW, et al. Unsupervised modeling and genome-wide association identify novel features of allergic march trajectories. J Allergy Clin Immunol. 2021;147(2):677-685 e610.
18. Del Pozo DV, Zhu Y, Mitra N, Hoffstad OJ, Margolis DJ. The risk of atopic comorbidities and atopic march progression among Black and White children with mild-to-moderate atopic dermatitis: A cross-sectional study. J Am Acad Dermatol. 2022.
19. McCormick JP, Lee JT. Insights into the Implications of Coexisting Type 2 Inflammatory Diseases. J Inflamm Res. 2021;14:4259-4266.
20. Senna G, Micheletto C, Piacentini G, et al. Multidisciplinary management of type 2 inflammatory diseases. Multidiscip Respir Med. 2022;17(1):813.
21. Fiocchi A, Vickery BP, Wood RA. The use of biologics in food allergy. Clin Exp Allergy. 2021;51(8):1006-1018.
22. Gallagher A, Edwards M, Nair P, et al. Anti-interleukin-13 and anti-interleukin-4 agents versus placebo, anti-interleukin-5 or anti-immunoglobulin-E agents, for people with asthma. Cochrane Database Syst Rev. 2021;10:CD012929.
23. Matucci A, Vivarelli E, Nencini F, Maggi E, Vultaggio A. Strategies Targeting Type 2 Inflammation: From Monoclonal Antibodies to JAK-Inhibitors. Biomedicines. 2021;9(10).
24. Gabryszewski SJ, Dudley J, Grundmeier RW, Hill DA. Early-life environmental exposures associate with individual and cumulative allergic morbidity. Pediatr Allergy Immunol. 2021;32(5):1089-1093.
25. Paller AS, Spergel JM, Mina-Osorio P, Irvine AD. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J Allergy Clin Immunol. 2019;143(1):46-55.
26. Amat F, Soria A, Tallon P, et al. New insights into the phenotypes of atopic dermatitis linked with allergies and asthma in children: An overview. Clin Exp Allergy. 2018;48(8):919-934.
27. Hassoun D, Malard O, Barbarot S, Magnan A, Colas L. Type 2 immunity-driven diseases: Towards a multidisciplinary approach. Clin Exp Allergy. 2021;51(12):1538-1552.
28. Boguniewicz M, Eichenfield LF, Hultsch T. Current management of atopic dermatitis and interruption of the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S140-150.
29. Gabryszewski SJ, Hill DA. One march, many paths: Insights into allergic march trajectories. Ann Allergy Asthma Immunol. 2021;127(3):293-300.