PAID POST: The following content is paid for by an advertiser. NEA doesn’t validate, endorse or fact check any claims made in paid advertising, nor is the content written by NEA.
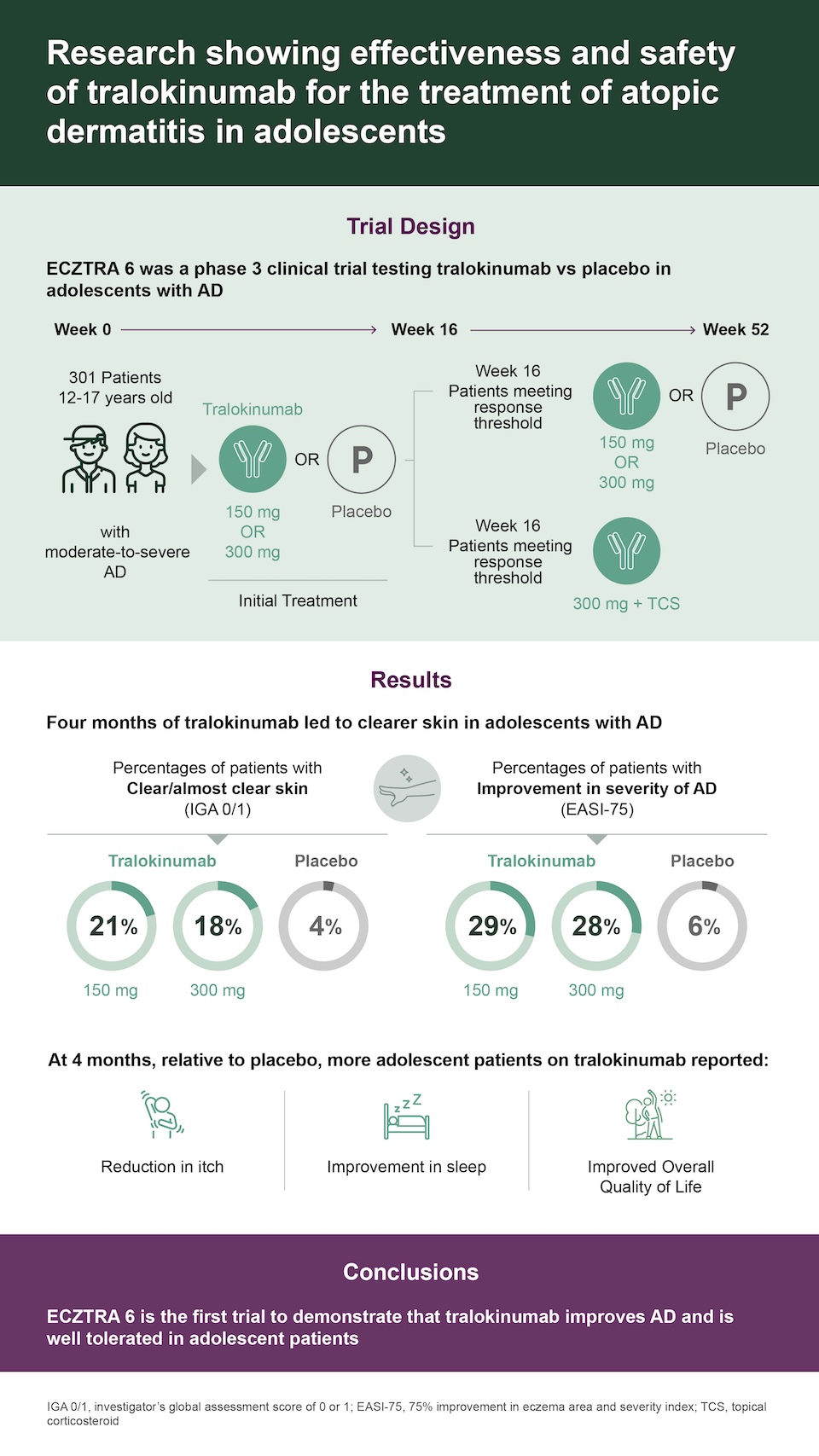
Atopic dermatitis (AD), or eczema, is a chronic disease causing painful, dry, itchy skin, which can negatively affect sleep, quality of life, and school performance. AD is caused by a combination of weakened skin barrier, altered immune system response, and imbalance in the common bacteria living on the skin surface. Tralokinumab is a medication targeting interleukin (IL)-13, a protein that drives the overactive immune response in AD. The ECZTRA 6 clinical trial tested the ability of tralokinumab to improve AD severity in adolescents.
Adolescents with moderate-to-severe AD received tralokinumab or nonactive drug (placebo). Patients meeting a threshold of improvement in AD severity after 16 weeks either continued treatment or received less frequent dosing for 8 additional months. Patients not meeting the target response (“non-responders”), received tralokinumab with optional use of topical corticosteroids. The medical team monitored patients and evaluated changes in AD symptoms and severity.
ECZTRA 6 was the first trial to demonstrate that tralokinumab improves AD and is well tolerated in adolescent patients. These findings support tralokinumab as an important long-term treatment option for adolescents with moderate-to-severe AD.